Background
As the antivaccine movement has increased, vaccine preventable illnesses have also increased. The resurgence of measles globally has been noted, specifically the United States received 1200 new cases of measles in 2019, which is the highest number of cases since 1994. Measles is a highly contagious pathogen; the virus has an attack rate of up to 90% in susceptible individuals. Standard of care for patients post hematopoietic cell transplant (HCT) includes repeating all childhood vaccinations. However, compliance with recommendations is unknown. No previous reports have been created measuring the difference in measles, mumps, and rubella (MMR) reactivity between autologous and allogeneic HCT patients post treatment.
Methods
A retrospective chart review investigated HCT patients between 2000-2019 from the Windsor Regional Hospital (WRH) cancer centre. Patients were excluded from data collection if they were deceased before an MMR reactivity test could be done or if they were lost to follow up. A total of 83% of autologous HCT (N=57) and 66% of allogeneic HCT (N=47) patients had serology tested to measure MMR reactivity post-transplant prior to live vaccinations. MMR titres were drawn from autologous patients a median of 395 days after HCT and a median of 907 days after HCT for allogeneic patients.
Results
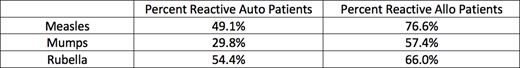
Overall, allogeneic HCT patients had more reactivity than autologous HCT patients as seen in Table 1.
Conclusion
All patients not reactive to measles need to be re-vaccinated 24 months after treatment according to 2019 American Society for Transplantation and Cellular Therapy guidelines. Our patients are treated in a variety of transplant centers in Ontario, Canada as well as Detroit, Michigan; which is of concern because measles cases have been reported in Detroit. The majority (66%) of allogeneic HCT patients had a myeloid malignancy, while 70% of autologous patients has a diagnosis of multiple myeloma. As evidenced by the poor MMR response, less robust immune systems may be accounted for by multiple myeloma patients. This does emphasize the need for MMR vaccinations post HCT for multiple myeloma patients and raises the question regarding immunization for at risk non-transplant eligible patients. We suggest that it may be reasonable to assess MMR immune status in all myeloma patients to determine if this is a transplant effect or an effect of the underlying immune status of the myeloma patient. This study does emphasize the need to assess MMR status post transplant in all myeloma patients, as the vast majority in our study did not demonstrate immunity post stem cell transplant.
Hamm:Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal